Well, if I told you that, I’d be misleading you and putting you at risk of an unintended pregnancy.
But, that’s what a company called Valley Electronics AG has been telling consumers, in hopes of selling their $330 device called Daysy.
The commentary examines a paper published in March 2018 by Dr. Martin Koch and colleagues, which purported to measure the contraceptive effectiveness of Daysy and the DaysyView app. Unfortunately, the analytic methods used in the Koch paper were flawed and inconsistent with accurate approaches to estimating effectiveness – making their results void of meaning.
Despite my prior efforts to express similar concerns directly and privately to the company regarding their concerning marketing language, their use of misleading language continued. So, in addition to detailing analytic concerns with the Koch analysis, the commentary describes these prior efforts to communicate concerns and provides examples of the misleading marketing language used by Valley Electronics.
Since the Koch estimates are unreliable (as both peer reviewers of my commentary agreed), I called for a retraction of the Koch manuscript from the scientific literature - to align with best practices in publishing and to protect public health. I'm grateful to the journal (Reproductive Health) for publishing my commentary, and look forward to learning if they will decide to retract the misleading Koch paper.
- (Note: I do not know how long the process will take for the journal to decide whether or not to retract the Koch paper. I submitted the commentary stating my concerns to the journal on April 6, 2018. After peer review, it was accepted for publication on June 14. On June 16, I asked the journal if they planned to retract Koch, and was told on June 22 that they'd decide within approximately a week. However, I didn't hear back for several more weeks. I followed up via email on July 10, and never heard back, so I called by phone to ask on July 18. I am told an investigation is ongoing, and that the journal does not know when a final decision will be available. I hope a decision is made as soon as possible - every additional day that the Koch paper is in the scientific literature is another day that an unsuspecting consumer might place their confidence in the misleading Koch estimates, and potentially put themselves at risk of unintended pregnancy - and another day in which misinformation can spread).
It is unacceptable for an unsuspecting public to be sold an inadequately tested device. In addition to correcting the scientific record, I hope regulatory authorities will investigate Valley Electronics concerning marketing claims.
To my knowledge, Daysy is sold in about 35 countries around the world. If you notice that a company is marketing uncleared or unapproved products in the United States, or using misleading promotional materials, you can report allegations to the FDA at this link.) People in other countries can contact their appropriate regulatory agency to file a complaint, too.
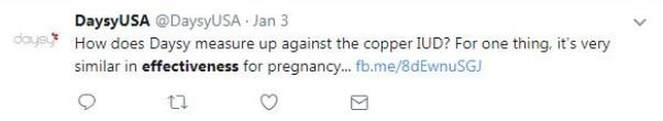
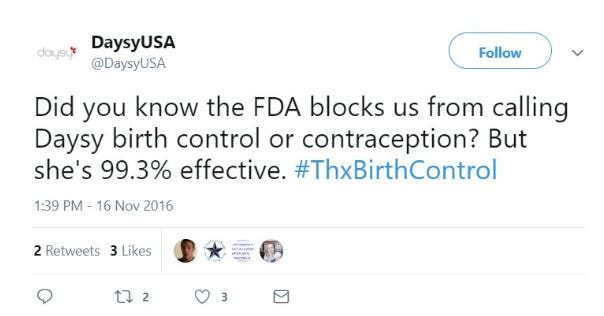
This is a 2016 Tweet from the company that gives a sense of their terrible ethics:
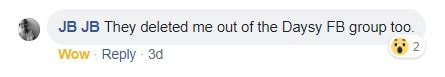
In the days since releasing my commentary, I have been deeply encouraged that multiple women interested in and/or using fertility awareness based methods (FABMs) or natural family planning (NFP), including some Daysy customers, have read and digested the commentary, and started to ask appropriate questions of Valley Electronics. Some people told me that in response, Valley Electronics kicked them out of private online forums pertaining to Daysy, and also shut down the ability to comment on threads in which questions are being asked (screenshots below shared with permission). This does not bode well for establishing trust with their customers or establishing transparency.
Ultimately, I hope this commentary helps to generate healthy discussion among reproductive health researchers and care providers, FABM/NFP educators, FABM/NFP users, and those with regulatory expertise. The scientific and medical community could think more critically about how to respond to a situation in which some members of the public feel more comfortable trusting claims from companies like Valley Electronics, than they do trusting those with expertise in estimating effectiveness of, and providing evidence-based information on, all options for pregnancy prevention.
UPDATE 7-4-18:
Somebody reached out to me to say that Daysy has released this statement about their study.
It's not entirely clear to me if the statement is supposed to be in response to my commentary, since it does not actually engage with the substantive methodological concerns raised, or rebut them in a way that would make any contraceptive effectiveness expert place more confidence in their estimates. If this is their response to my commentary, their customers and the general public should be even more alarmed.
The statement contains double-speak. For example, it continues to call the study an "independent clinical study" but admits that Dr. Niels van de Roemer (Director of Medical Affairs for Valley Electronics), "analyzed the stored data". Having an employee of the company that manufactures the device be the one responsible for analyzing some or all of the data does not constitute an "independent" study (nor does the study design constitute a "clinical study").
The statement admits that "Daysy is not a contraceptive in the real sense". This message is in stark contrast to how they market the device (selling it as a contraceptive method, claiming a certain level of contraceptive effectiveness, and saying things in the Koch 2018 paper like "The contraceptive effectiveness of the fertility monitor (Daysy) has already been demonstrated in an independent trial.") They mislead consumers with this kind of double-speak.
The paper and statement note that the "data provided by participants were double checked for their correctness from comparison with the internal database. With this measure, it could be further ensured that no doubts had taken part in the survey." This is gobbledygook. What was this magical "internal database" that enabled them to confirm all of the information collected in the survey? And why didn't they use the magical internal database in the first place, instead of going to the trouble of doing a survey? The Daysy device itself collects only temperature and menstrual cycle dates. Saying there could be "no doubts" makes clear that these authors do not understand surveys in general, let alone the important concern that unintended pregnancies are often under-reported in surveys.
The statement concludes by saying that the Valley Electronics team "prides itself on professionalism, transparency, and integrity" - which is doubly shocking given all that I describe above on how they are silencing and blocking customers who are asking questions.
I also note that they refer to "literature" and list 8 sources. A few points:
- Most titles listed pertain to BabyComp, LadyComp, or Pearly. These devices are ancestors of Daysy, and it is unclear exactly how they differ from Daysy. It does not make sense to list them as evidence on Daysy effectiveness.
- Titles of some of the papers listed don't even pertain to effectiveness for pregnancy prevention. For example, one is called "Monitoring of ovulation by sophisticated digital electronic BBT recording "Babycomp" in patients with unexplained infertility" - a study on a topic irrelevant to the issue at hand. Listing these here does not bolster their scientific credibility, it makes them look more incompetent and more insulting to the intelligence of their customers and the public.
- I've read three of the items listed - Koch 2018 (the focus of my commentary), Freundl 1998 (addressed briefly in my commentary), and Demianczyk 2016 (so bad as to be practically illegible) - none provide reliable information on Daysy effectiveness.
- Note that this list does not indicate the journals in which this "literature" is published. I've been looking in the academic literature trying to locate some of these sources. At least some seem to be conference abstracts, and not peer reviewed publications.
Valley Electronics is digging itself into a deeper hole with documents like these, and continuing to endanger the health and well-being (not to mention trust) of its customers.
At this time, we await word from Reproductive Health on whether they plan to retract the Koch manuscript.
________________________________________________________________________________________
NOTE: While the commentary is specific to Daysy, I'd like to provide a few resources more broadly on FABMs/NFP, for folks who may be less familiar with these methods.
- Multiple kinds of FABMs exist (see our recent webinar for an overview), and each uses different markers of fertility to identify the days in each menstrual cycle when conception is most likely if unprotected penile/vaginal sex occurs.
- About 3% of female contraceptors in the US report using an FABM, and among those, most report using the rhythm method.
- FABMs have received increased attention recently in the political arena, in part because the Trump administration appears to be prioritizing them over all other contraceptive options – at the expense of full contraceptive choice and client-centered medical care. People should have robust information about and access to all of their contraceptive options, and should be supported in making a decision on which method feels right for themselves.
- This blog from last year aims to help people better understand some of the existing effectiveness estimates for fertility awareness based methods of contraception.
- Our systematic review on the effectiveness of all FABMs as evaluated in prospective studies was recently published in the journal Obstetrics and Gynecology. A pdf of the study is freely available at this link through the end of September.
- Check out our new webinar on effectiveness of fertility awareness based methods
- Here are a few recent media pieces that mention the Daysy situation
 RSS Feed
RSS Feed